Assuming 100 Dissociation Which of the Following Compounds
Vant Hoff factor i is given by the expression. Moles of solute 063 mol solute 100 kg solvent 00550 kg solvent 0035 mol.

Calculate Molarity Of All The Ions Produces By Following Aq Solution Of Electrolytes Assuming 100 Dissociation I 0 2 M Nacl Solution Ii 1 2 M Al 2 So 4 Solution Iii 1 2 M H 2 So 4 Solution Vi
View the full answer.
. Assuming 100 dissociation calculate the freezing point and boiling point of 313 m SnCl4aq. The difference between the boiling point and the freezing point of pure water at standard pressure is 132 K 2273 K 3100 K 4373 K Chemistry Assuming complete dissociation of the solute how many grams of KNO3 must be added to 275 mL of water to produce a solution that freezes at -145 degrees celcius. It is important to be able to write dissociation equations.
Calculate the freezing point of the following solutions assuming complete dissociation37 KCL by mass in water. Al2 SO43 i 4 NH4NO3 i 2 Mg NO32 i 3 Na2SO4 i 3 Sucrose i 1. 2000 wt C 2664wt O 4665 wtN and 671wtH.
Assuming 100 dissociation which of the following compounds has an incorrectly listed vant Hoff factor. Which of the following is an ionic compound that dissociates in water. For 100 dissociation.
Therefore Vant Hoff factor is 2. CaClO32 i 2 NH42SO4 i 3 NH43PO4 i 5 Urea i 2 Sc2SO43 i 6. 3 2 i 2.
Tf soln Tf solvent - m x kf kf for water is -186 Cm and Tf for water is 0 C Tf soln 0 C - 328 m x 186 Cm Tf soln -61008 C Boiling point. Compounds Olonic compounds with no polyatomic ions Acid and bases Question 5 1 pts If a certain mass of an ionic solute is added to a solvent such as water which of the following boiling points is reasonable for. MgNO32 i 2 NH4NO3.
And 0030 mol of MgCl 2 assuming complete dissociation of these electrolytes. How many moles of ions are produced by the dissociation of 1 mol of NH4Br. The subscripts for the ions in the chemical formulas become the coefficients of the respective.
B NH 4 2SO 4 i 3 C NH 4 3PO 4 i 5 D Urea i 2 E Sc 2SO 4 3 i 6. Refer to following figure for the following three questions. When NaCl dissociates completely it gives two ions Na and Cl.
3 Assuming 100 dissociation which of the following compounds is listed correctly with its vant Hoff factor A Ca CIO32 i 2 B NH42SO4 i 3 C NH43PO4 i 5 D Urea i 2 E Sc2 SO43 i 6 Answer. Assuming 100 dissociation calculate the freezing point and boiling point of 244 m Na2SO4aq. Each of the following compounds form.
I - 2 Al2SO43. Assuming 100 dissociation calculate the freezing point Tf and boiling point Tb of 133 m AgNO3aq. K2SO4 i 3 b.
If the vapor pressure of pure water at 25 C is 237 torr what is the vapor pressure of the solution. Think of the number of ions that each compound is made of is it 2 3 4 D Al2SO43 i 4. 19Assuming 100 dissociation which of the following compounds is listed correctlywith its vant Hoff factor i.
Chemistry questions and answers. Colligative constants can be found in the chempendix. Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor i.
Kb for water is 0512 kb of water is 100 C Tb soln Tb solvent - kb x m Tb soln 100 0512 x 328 Tb soln 10168 C. However for some weak acids the percent dissociation can be higherupwards of 10 or more. Moles of solute 063 mol solute 100 kg solvent 00550 kg solvent 0035 mol.
Hence option B is correct. Predict which compound in each pair will. Select all that apply.
How many moles of ions are produced by the dissociation of 1 mol of MgCl2. Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor i. The freezing point depression approaches the value calculated by assuming.
Write the following in order of increasing melting point Cl2 I2 Br2 F2 F2 Cl2 Br2 I2. Glucose I 2. Determine the number of moles of compound in the solution from the molal concentration and the mass of solvent used to make the solution.
Assuming 100 dissociation which of the following compounds is listed correctly with its vant Hoff factor i. A compound was found to be composed of the following amounts of elements. Simply undo the crisscross method that you learned when writing chemical formulas of ionic compounds.
Assuming 100 dissociation which of the following compounds is listed correctly with its vant Hoff factor i. NH4NO3 i 2 c. SnCl4 with 100 dissociation gives a vant hoff factor of 5 1Sn 4 ion and 4 Cl- ions deltaT i x Kf x m for freezing point 5 x 051Cm x.
A Na b I2 c CO2. NH42SO4 i 3 Calculate the boiling point of a 45 m solution of Na2SO4 in water assume 100 dissociation Kb H2O 052 Cm. Means How many mEq each molecule forms a.
For example with a problem involving the percent dissociation of a 0100 M chloroacetic acid we cannot assume x is small and therefore use an ICE table to solve the problem. A nonvolatile compound dissolved in 0100 kg of water. Sucrose i 1 d.
Assuming 100 dissociation 10 mol of NaBr dissolved in 250 g of water Select have the same colligative properties as 10 mol. I - 5 Na2SO4 1-3. 4 A solution of chloroform CHCl3 and acetone CH32CO exhibits a negative deviation from Raoults law.
O Sucrose 1 1 O NH4NO3 i 2 O Mg NO32 i 3 O Al2 SO43 i. As we would expect for a weak acid the percent dissociation is quite small. The Vant Hoff factor of N a C l assuming 100 dissociation is.
For which of the following solutes the vant Hoff factor is not greater than one. In which of the following solvents would you expect KBr to be most soluble. A Metallic b Molecular c Molecular.
T C Ть. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor i.
Assuming 100 dissociation calculate the freezing point T and boiling point T of 313 m KPO aq. Assuming 100 dissociation which of the following compounds is listed incorrectly with its vant Hoff factor i.

Ph Log H Assuming 100 Percent Dissociation If Given Percent Ionization Multiply By The Molarit Chemistry Education Chemistry Lessons Chemistry Classroom

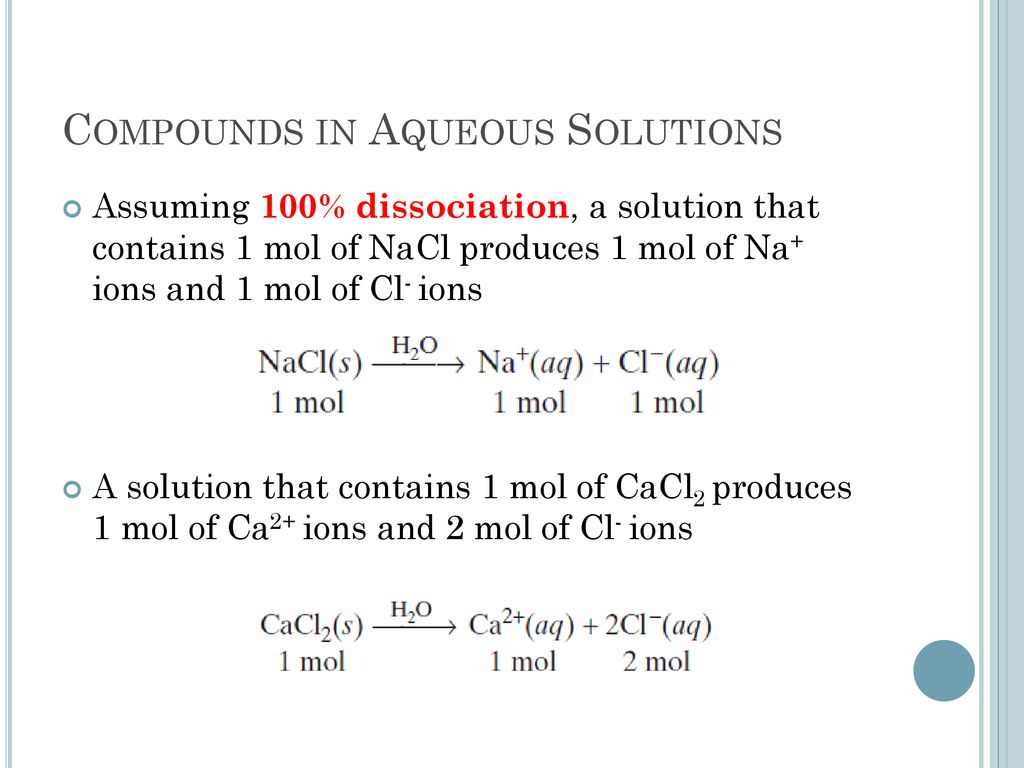
Ions In Aqueous Solutions Ppt Download

Ions In Aqueous Solutions And Colligative Properties Ppt Video Online Download

Comments
Post a Comment